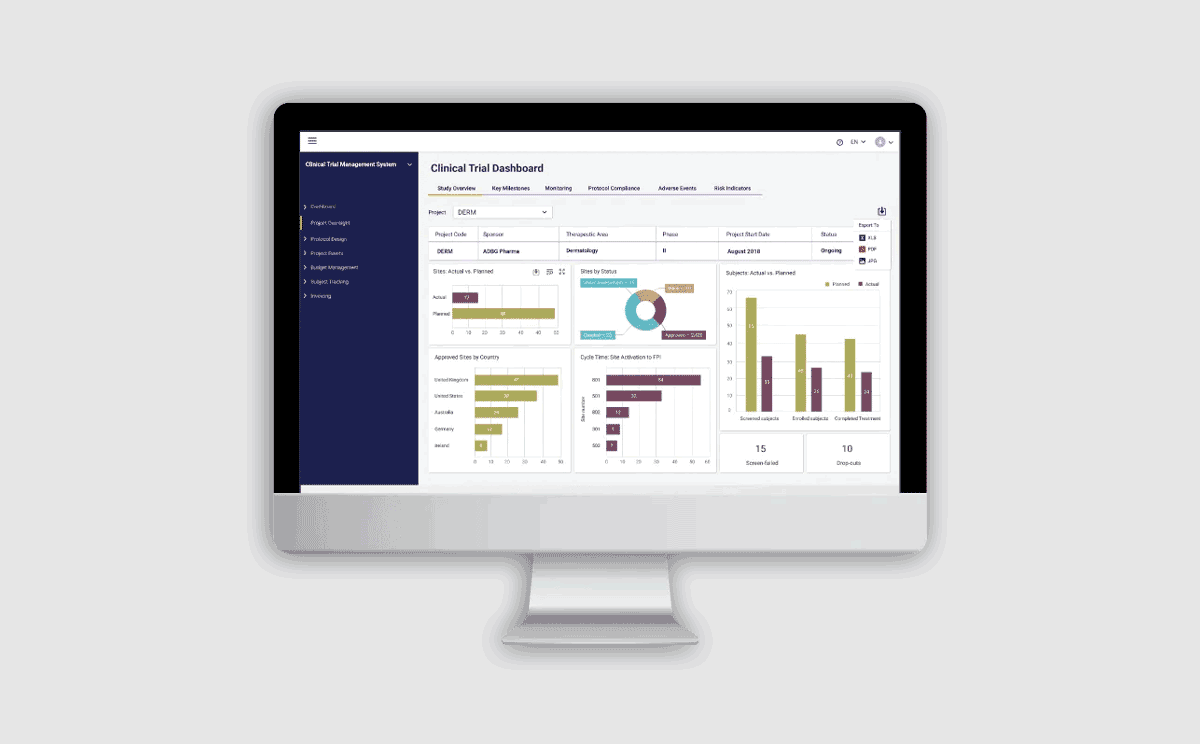
Get in Touch

You're just a message away from turning "what if" into "let's do it!"
Time zone

12:00 AM Next
12:30 AM Next
01:00 AM Next
01:30 AM Next
02:00 AM Next
02:30 AM Next
03:00 AM Next
03:30 AM Next
04:00 AM Next
04:30 AM Next
05:00 AM Next
05:30 AM Next
06:00 AM Next
06:30 AM Next
07:00 AM Next
07:30 AM Next
08:00 AM Next
08:30 AM Next
09:00 AM Next
09:30 AM Next
10:00 AM Next
10:30 AM Next
11:00 AM Next
11:30 AM Next
12:00 PM Next
12:30 PM Next
01:00 PM Next
01:30 PM Next
02:00 PM Next
02:30 PM Next
03:00 PM Next
03:30 PM Next
04:00 PM Next
04:30 PM Next
05:00 PM Next
05:30 PM Next
06:00 PM Next
06:30 PM Next
07:00 PM Next
07:30 PM Next
08:00 PM Next
08:30 PM Next
09:00 PM Next
09:30 PM Next
10:00 PM Next
10:30 PM Next
11:00 PM Next
11:30 PM Next

Review & Confirm
 30 Min
30 Min


You are scheduled
A calender invitation has been send to your email.
30 Min Meeting
 30 Min
30 Min